True Cell Culture & Perfusion Control
Perfusion
Perfusion, if well managed, offers many economical and performance advantage.
Biologically, the perfusion principle is a steady benefit for the following reasons:
- No accumulation of biological waste
- Proteins prone to instability are quickly removed
- Cells grow more naturally with less stress
- Recombinant proteins/ mAbs are usually purer
- Proteins obtained are more biologically consistent, with fewer glycosylation variations
- The cost of any batch failures tends to be lower than with fed-batch
Unfortunately, perfusion is often perceived as too complex, and perfusion devices thought to be too expensive and likely to clog; achieving higher productivity without a long, costly investment and optimization of the process and scale-up are seen as difficulties; people believe no process control can truly manage perfusion; and,finally, it is said that set-up is too long, complex, and has too many risks of contamination if you are not a perfusion expert.
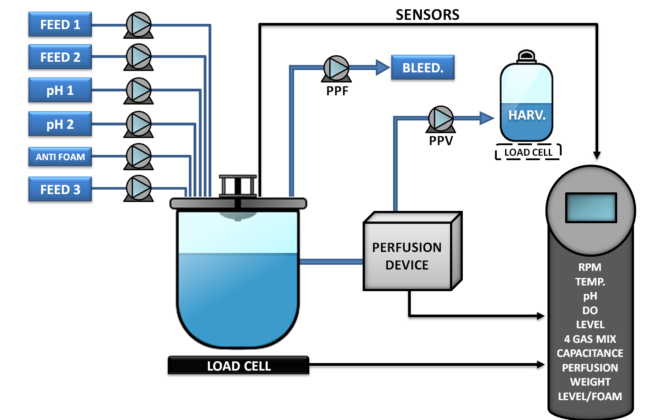
CYTOSYS is an automated capacitance-based, software-driven compact system allowing mammalian cells to be grown under perfusion conditions with continuous monitoring of cell concentration between 2 and several millions of cells/ml. Perfusion is fully monitored using the CYTOPERFUSION model (see diagram below). Cell concentration is controlled with capacitance, and perfusion rate is managed remotely using a proprietary algorithm.
Perfusion is then better controlled, productivity is enhanced, and quality from batch to batch remains universally excellent.
Perfusion with it's unique capability makes the following tasks easier
Production of cells
Production of cells with similar quality at low, medium or high cell concentration.
Easy for work
Less complex human intervention during the process.
Rapid maximization
Process is equally well managed and controlled with or without local human supervision.
Rapid optimization
Rapid optimization of a process, compared to classic methods.
Optimized for you
Scale-up is less influenced by engineering issues.
Detection of contamination or process deviation
Very early detection of any contamination or process deviation
- Freedom to operate any type of benchtop glass vessel or single use bioreactors
- Freedom to integrate the preferred type of capacitance sensing to run perfusion
- Freedom to use any type of similar or different perfusion devices at same time
- Retrofit to an existing bioreactor or to a new equipment project
- Quick adaptation to a process with performance demonstrated after first run .
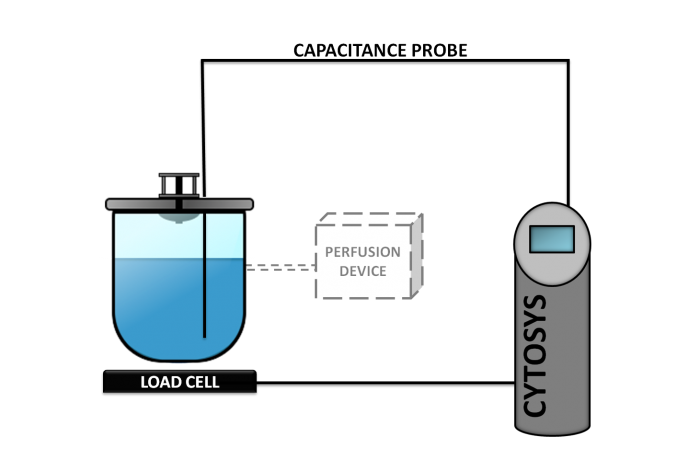
Phase 1
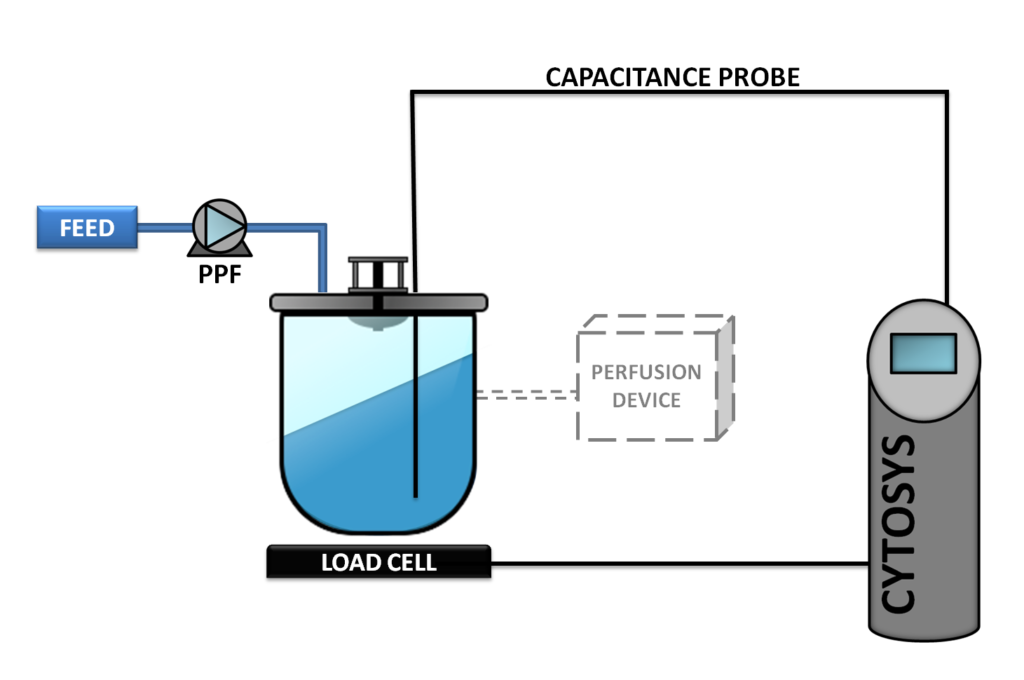
Phase 2
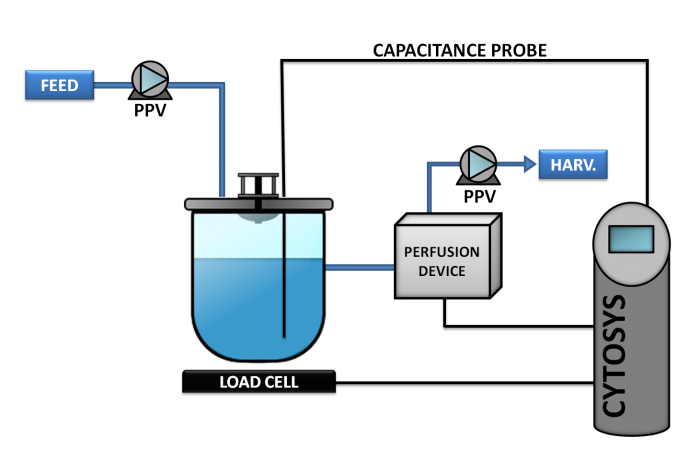
Phase 3
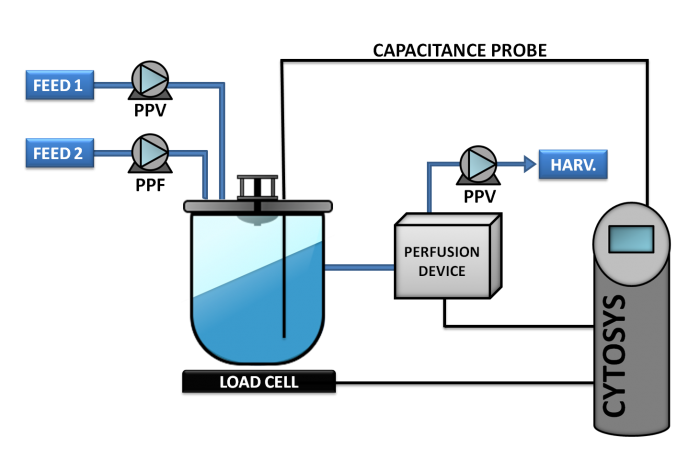
Phase 4
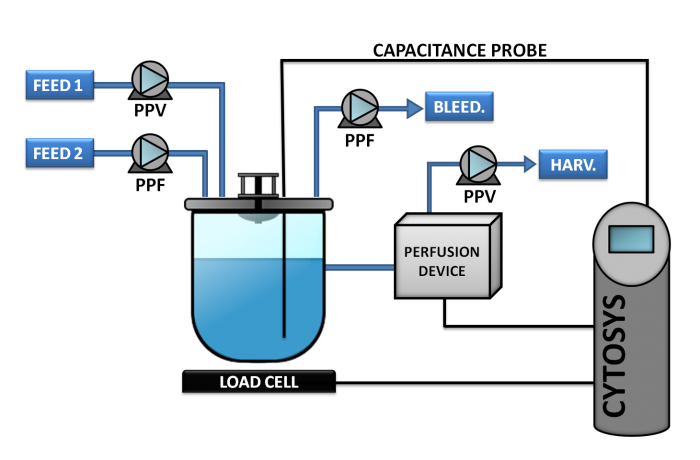
Phase 5


This concept, also known as CYTOPERFUSION, controls the entire process from inoculation to the steady state conditions and continuous production at the cell concentration targeted. Process is then maintained for the time required, from a week to as long as nine months.
